ground state electron configuration for cr2+|Spectroscopic Ground States : Tuguegarao In order to write the Mg electron configuration we first need to know the . Floaty crowny things, Odd parents, fairly odd parents, Really mod, pea pod, buff bod, hot rod, [Timmy] Obtuse, rubber goose, green moose, guava juice, Giant snake, birthday cake, large fries, chocolate shake. Odd parents, fairly odd parents, It flips your lid when you are the kid, With fairly odd parents.
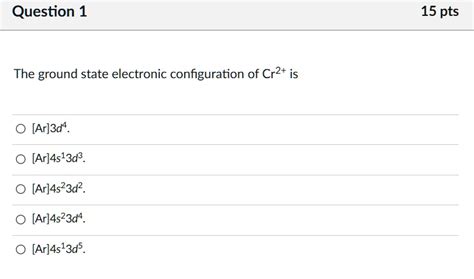
ground state electron configuration for cr2+,1s2 2s2 2p6 3s2 3p6 3d5 4s1. For the Cr 2+ ion we remove one electron from 4s1 and one from the 3d5 leaving us with: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4. For the Cr 3+ ion we remove a total of three electrons (one from the 4s1 and two from the 3d5) leaving us with. 1s 2 2s .Correct Electron Configuration for Copper (Cu) Half-filled and fully filled subshell .Therefore the N electron configuration will be 1s 2 2s 2 2p 3. Video: Nitrogen .In order to write the Mg electron configuration we first need to know the .
We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since .ground state electron configuration for cr2+Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s . 1s22s22p63s23p64s13d5. And the electrons in the 4s orbital is removed first because this orbital lies further from the nucleus, making electrons easier to remove in . 205K views 4 years ago Electron Configurations. To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to. To write the electron configuraiton for a cation, start by writing the electron configuration for the neutral atom. Then determine the number of electrons that were .
There are two unpaired electrons in this system from the electron configuration. Rule 1 predicts that the ground state will be a triplet with \(S=1\) so .
Write the expanded and shortened ground state electron configuration for Cr. Write the expanded and shortened ground state electron configuration for Cu. Write .ground state electron configuration for cr2+ Spectroscopic Ground States Experimentally, we observe that its ground-state electron configuration is actually [Kr]5s 1 4d 4. We can rationalize this observation by saying that the electron–electron . Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be .
Sz = (+1, 0, −1), (0) or. S = 1, 0. Multiplicity = (2S + 1) = 3 and 1. The multiplicity actually represents the number of orientations possible for the total spin relative to the total orbital .
Rule 2 predicts a F F state since that is the highest multiplicity with L = 3 L = 3: So the ground state is from this more narrowed list: 3F4 3 F 4, 3F3 3 F 3, 3F2 3 F 2. Rule 3 predicts the lowest J J term since the d shell is less than half full. That is the J = 2 J = 2 state. Therefore for this system, the atom will have a ground-state .
Sarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is .
Question: The ground-state electron configuration of a Cr* ion is 1s 2s 2p 3s 3p 3d". Therefore, Cr2+ is Answers: A. diamagnetic B. paramagnetic with one unpaired electron. C. paramagnetic with three unpaired electrons. D. paramagnetic with four unpaired electrons. E. paramagnetic with five unpaired electrons. There are 2 steps to solve this one.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer. Question: Predict the ground‑state electron configuration of each ion. Use the abbreviated noble gas notation. Cr2+ : Cu2+ : Co3+ : Predict the ground‑state electron configuration of each ion. Use the abbreviated noble gas notation.This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Write the complete ground-state electron configuration of Cr2+. For multi-digit superscripts or coefficients, use each number in succession. There are 2 steps to solve this one.
In several cases, the ground state electron configurations are different from those predicted using th periodic table and the Aufbau Principle. Some of these anomalies occur as the 3d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4s 1 3d 5 rather than the predicted [Ar]4s 2 3d 4.The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).Figure 6.29 shows the lowest energy, or ground-state, electron configuration for these elements as well as that for atoms of each of the known elements. Figure 6.29 This version of the periodic table shows the outer-shell electron configuration of each element. Note that down each group, the configuration is often similar.Chemistry questions and answers. Write ground-state electron configurations for the ions sr, cr, Al*and Cr2*. Which do you expect will be paramagnetic due to the presence of unpaired electrons? (Express your answer as a series of orbitals. For example, the electron configuration of Li would be entered in complete form as 1s* 2s1 or in condensed .

Here are the ground state configurations: Cr2+: The neutral chromium atom has an electron configuration of [Ar]3d54s1. Upon losing two electrons to form Cr2+, it has [Ar]3d4. Cu2+: Neutral copper has the electron configuration [Ar]3d104s1. As Cu2+, it loses two electrons resulting in [Ar]3d9. Co3+: Neutral cobalt has [Ar]3d74s2, and upon .The ground-state electron configuration of Cr2+ is [Ar]4s13d3 none of the responses is correct [Ar]4s23d5 [Ar]4s23d2 O [Ar]3d4 This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.VIDEO ANSWER: Hi, in this question they have asked about the unpaired electrons present in chromium 3 plus ion right. So let's write the electronic configuration for chromium that is 24. So it is 1s2, 2s2, 3s2, 3b6, 3d5 and 4s1. If you write theFor instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The calcium ion (Ca 2+), however, has two electrons less. Hence, the electron configuration for Ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Since we need to take away two electrons, we first remove electrons from the outermost shell (n=4). Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Write the complete ground-state electron configuration of Cr2+. Write the complete ground-state electron configuration of zirconium. Here’s the best way to solve it.
PROBLEM 3.1.12 3.1. 12. In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co 2+ and Co 3+. Write the electron structure of the two cations. Answer.

The correct statement (s) about Cr2+ and Mn3+is (are) \lbrack Atomic numbers of Cr 24 and Mn 25\rbrack (A) Cr2\ast is a reducing agent (B) Mn3+ is an oxidizing agent (C) Both Cr2+ and Mn3+ exhibit d4 electronic configuration (D) When Cr2+ is used as a reducing agent, the chromium ion attains d5 electronic configuration. View Solution.Spectroscopic Ground States The correct statement (s) about Cr2+ and Mn3+is (are) \lbrack Atomic numbers of Cr 24 and Mn 25\rbrack (A) Cr2\ast is a reducing agent (B) Mn3+ is an oxidizing agent (C) Both Cr2+ and Mn3+ exhibit d4 electronic configuration (D) When Cr2+ is used as a reducing agent, the chromium ion attains d5 electronic configuration. View Solution.1. Table 0.1.2.1.1 0.1.2.1. 1. Four considerations in predicting ground state electron configuration of multi-electron atoms and ions. (1) Electrons will occupy the lowest energy orbitals in order to minimize the total energy. The two quantum numbers that are related to energy in multi-electron atoms are n n, and l l.
ground state electron configuration for cr2+|Spectroscopic Ground States
PH0 · What is the electron configuration of #"Cr"^(2+)#
PH1 · What is the electron configuration of "Cr"^(2+) ?
PH2 · Spectroscopic Ground States
PH3 · Electron Configuration for Cr, Cr2+, and Cr3
PH4 · Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH5 · Electron Configuration for Chromium (Cr and Cr2+, Cr3+ ions)
PH6 · Electron Configuration for Chromium (Cr and Cr2+, Cr3
PH7 · Electron Configuration Calculator
PH8 · 8.10: Hund's Rules Determine the Term Symbols of the
PH9 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH10 · 1.9A: Ground State Electronic Configurations
PH11 · 1.7: How to Write a Ground State Electron Configuration